PenMed’s team of MedTech innovation specialists provide full-stack, embedded systems development.
Our team of specialist experts.
Our team comprises experts with diverse backgrounds and extensive experience in medical technology, engineering, and business strategy. We are dedicated to driving innovation and delivering cutting-edge solutions in the MedTech, Surgical, and Diagnostics sectors.
Our in house team works closely with our trusted partners & clinicians, to collaborate & extend our reach.
Our Story.
PenMed emerged during the UK Ventilator Challenge, where Swagelok Bristol and Dr. Seb Brown collaborated to design and manufactured 200 ventilators in just four months. This remarkable achievement led Dr. Seb Brown and Simon Cooke to establish PenMed as an independent company.
Building on our initial success, PenMed now offers a comprehensive consultancy service. We provide clients with the additional expertise and flexible resources needed to transform their ideas and innovations into practical, real-world solutions. Our team is committed to delivering excellence through innovative design, rigorous engineering, and strategic planning.


Empowering Innovation in MedTech Development.
The medtech sector is undergoing a paradigm shift, with medical devices increasingly embedded within diagnostic, therapeutic, and care delivery ecosystems. Data-driven decision-making and system interoperability are now foundational to clinical workflows.
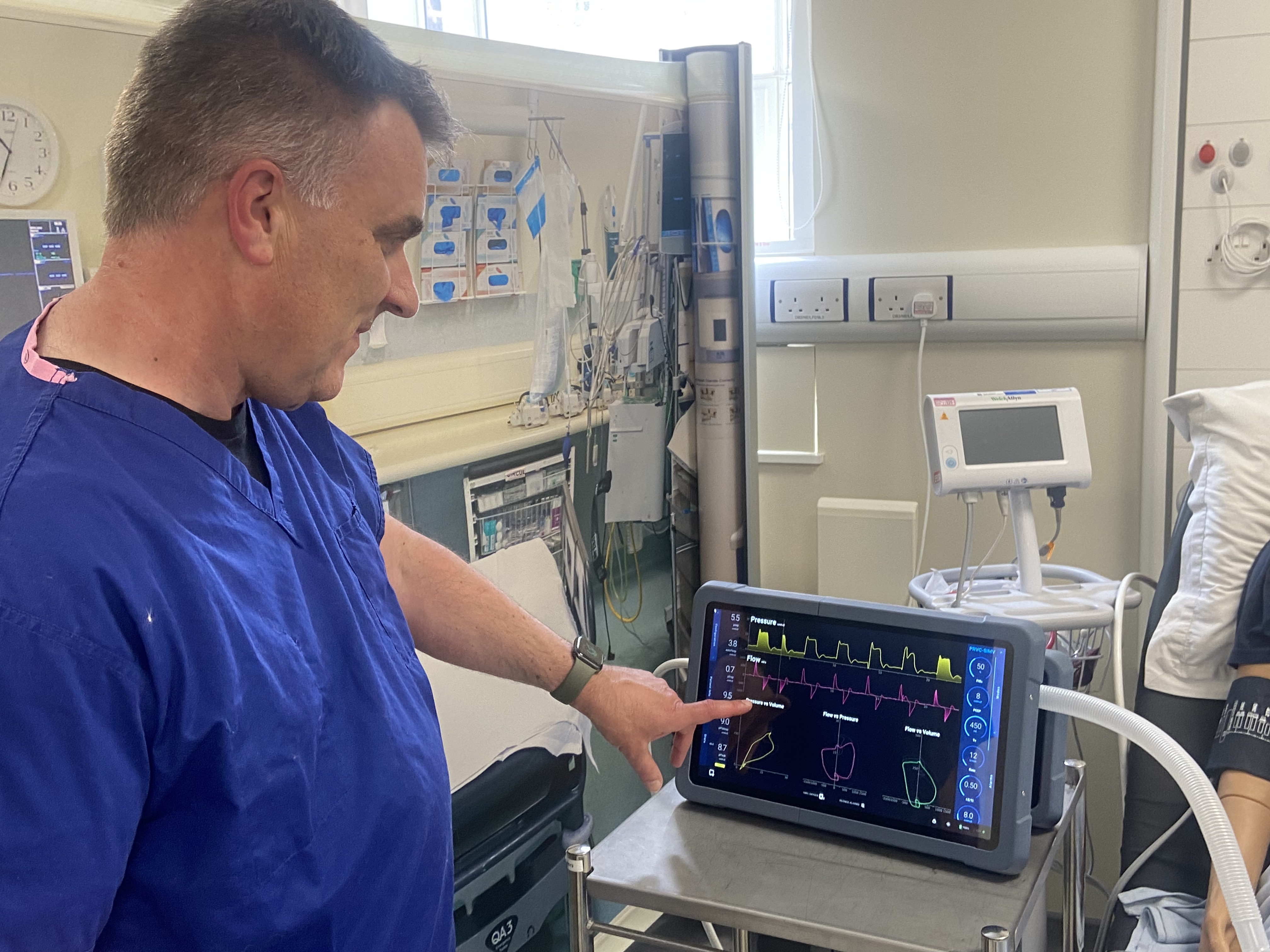
At PenMed, we deliver a full stack, embedded system development for critical care devices.
The PenMed Ventilator
From years of clinical experience and innovation in clinical care, the PenMed team has seen the risks associated with ventilators designed for specific environments along the care pathway. Patients being frequently disconnected from ventilators in one department and reconnected is far from ideal.
“A patient was transferred from theatre, post operatively on a portable ventilator. In the ICU they were connected onto an ICU ventilator, but quickly desaturated and cardiac arrest ensued. CPR was commenced and the ICU ventilator was found still to be in ‘stand-by’.”
Safety Incident in Critical Care – Bulletin March 2021
The PenMed ventilator has been developed by our specialist full-stack team, to provide a device that remains with the patient, in both trauma and surgical situations, along the full care pathway in all hospital departments.

We collaborate with a wide range of partners, from innovation labs and academic research teams to clinical specialists that practice in hospital.
Innovation & technology partners.






Academic & clinical partners.







Funders & enablers.